Azo synthesis meets molecular iodine catalysis†
Abstract
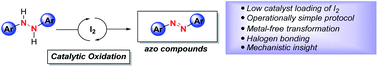
A metal-free synthetic protocol for azo compound formation by the direct oxidation of hydrazine HN–NH bonds to azo group functionality catalyzed by molecular iodine is disclosed. The strengths of this reactivity include rapid reaction times, low catalyst loadings, use of ambient dioxygen as a stoichiometric oxidant, and ease of experimental set-up and azo product isolation. Mechanistic studies and density functional theory computations offering insight into this reactivity, as well as the events leading to azo group formation are presented. Collectively, this study expands the potential of main-group element iodine as an inexpensive catalyst, while delivering a useful transformation for forming azo compounds.
- This article is part of the themed collection: How does it work? – a collection on mechanistic studies


 Please wait while we load your content...
Please wait while we load your content...