Synthesis of fully protected trinucleotide building blocks on a disulphide-linked soluble support†
Abstract
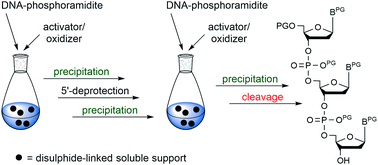
In recent years, preparation of fully protected trinucleotide phosphoramidites as synthons for the codon-based synthesis of gene libraries as well as for the assembly of oligonucleotides from blockmers has gained much attention. We here describe the preparation of such trinucleotide synthons on a soluble support using a disulphide linker.


 Please wait while we load your content...
Please wait while we load your content...