Ionic liquids and deep eutectic solvents for the recovery of phenolic compounds: effect of ionic liquids structure and process parameters
Abstract
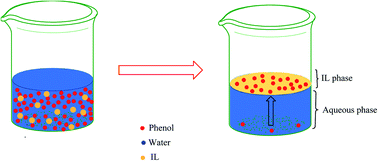
Water pollution is a severe and challenging issue threatening the sustainable development of human civilization. Besides other pollutants, waste fluid streams contain phenolic compounds. These have an adverse effect on the human health and marine ecosystem due to their toxic, mutagenic, and carcinogenic nature. Therefore, it is necessary to remove such phenolic pollutants from waste stream fluids prior to discharging to the environment. Different methods have been proposed to remove phenolic compounds from wastewater, including extraction using ionic liquids (ILs) and deep eutectic solvent (DES), a class of organic salts having melting point below 100 °C and tunable physicochemical properties. The purpose of this review is to present the progress in utilizing ILs and DES for phenolic compound extraction from waste fluid streams. The effects of IL structural characteristics, such as anion type, cation type, alkyl chain length, and functional groups will be discussed. In addition, the impact of key process parameters such as pH, phenol concentration, phase ratio, and temperature will be also described. More importantly, several ideas for addressing the limitations of the treatment process and improving its efficiency and industrial viability will be presented. These ideas may form the basis for future studies on developing more effective IL-based processes for treating wastewaters contaminated with phenolic pollutants, to address a growing worldwide environmental problem.
- This article is part of the themed collection: 2021 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...