Bonding and stability of donor ligand-supported heavier analogues of cyanogen halides (L′)PSi(X)(L)†
Abstract
Fluoro- and chloro-phosphasilynes [X–Si![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P (X = F, Cl)] belong to a class of illusive chemical species which are expected to have Si
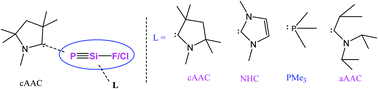
P (X = F, Cl)] belong to a class of illusive chemical species which are expected to have Si![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P multiple bonds. Theoretical investigations of the bonding and stability of the corresponding Lewis base-stabilized species (L′)PSi(X)(L) [L′ = cAACMe (cyclic alkyl(amino) carbene); L = cAACMe, NHCMe (N-heterocyclic carbene), PMe3, aAAC (acyclic alkyl(amino) carbene); X = Cl, F] have been studied using the energy decomposition analysis-natural orbitals for chemical valence (EDA-NOCV) method. The variation of the ligands (L) on the Si-atom leads to different bonding scenarios depending on their σ-donation and π-back acceptance properties. The ligands with higher lying HOMOs prefer profoundly different bonding scenarios than the ligands with lower lying HOMOs. The type of halogen (Cl or F) on the Si-atom was also found to have a significant influence on the overall bonding scenario. The reasonably higher value and endergonic nature of the dissociation energies along with the appreciable HOMO–LUMO energy gap may corroborate to the synthetic viability of the homo and heteroleptic ligand-stabilized elusive PSi(Cl/F) species in the laboratory.
P multiple bonds. Theoretical investigations of the bonding and stability of the corresponding Lewis base-stabilized species (L′)PSi(X)(L) [L′ = cAACMe (cyclic alkyl(amino) carbene); L = cAACMe, NHCMe (N-heterocyclic carbene), PMe3, aAAC (acyclic alkyl(amino) carbene); X = Cl, F] have been studied using the energy decomposition analysis-natural orbitals for chemical valence (EDA-NOCV) method. The variation of the ligands (L) on the Si-atom leads to different bonding scenarios depending on their σ-donation and π-back acceptance properties. The ligands with higher lying HOMOs prefer profoundly different bonding scenarios than the ligands with lower lying HOMOs. The type of halogen (Cl or F) on the Si-atom was also found to have a significant influence on the overall bonding scenario. The reasonably higher value and endergonic nature of the dissociation energies along with the appreciable HOMO–LUMO energy gap may corroborate to the synthetic viability of the homo and heteroleptic ligand-stabilized elusive PSi(Cl/F) species in the laboratory.


 Please wait while we load your content...
Please wait while we load your content...