Amino acid ester-coupled caffeoylquinic acid derivatives as potential hypolipidemic agents: synthesis and biological evaluation†
Abstract
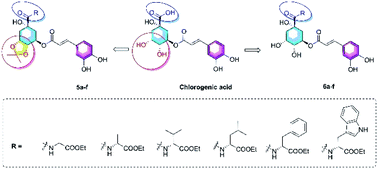
Pandanus tectorius (L.) Parkins. (PTPs) is rich in caffeoylquinic acids and amino acids, especially some essential amino acids, such as valine, phenylalanine, and so forth. A series of novel amino acid ester-coupled caffeoylquinic acid derivatives have been designed and synthesized. Biological evaluation suggested that some amino acid ester-coupled derivatives exhibited varying degrees of lipid-lowering effects on oleic acid-elicited lipid accumulation in HepG2 liver cells. Particularly, derivatives 6c, 6d, 6e and 6f exhibited comparable potential lipid-lowering effect with the positive control simvastatin and chlorogenic acid. Further studies on the mechanism of 6c, 6d, 6e and 6f revealed that the lipid-lowering effects were related to their regulation of TG levels and mRNA levels of lipometabolic-modulating genes, and merit further investigation.
- This article is part of the themed collection: 2021 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...