β-Cyclodextrin: a supramolecular catalyst for metal-free approach towards the synthesis of 2-amino-4,6-diphenylnicotinonitriles and 2,3-dihydroquinazolin-4(1H)-one†
Abstract
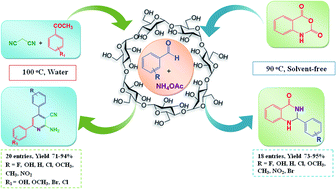
β-Cyclodextrin, a green and widespread supramolecular catalyst, has been explored as a highly proficient promoter for the metal-free one-pot multi-component synthesis of a vast range of highly functionalized bioactive heterocyclic moiety, 2-amino-4,6-diphenylnicotinonitriles and 2,3-dihydroquinazolin-4(1H)-one, from easily available precursor aldehydes. The main endeavor of these protocols is to explore this organic supramolecule in one-pot multi-component synthesis. Absence of metal catalyst or toxic acid and harsh reaction conditions, excellent functional group tolerance, inexpensive, greener and environmentally safe protocol are the key advantages of this work.
- This article is part of the themed collection: 2021 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...