Bridging-arylene effects on spectroscopic and photophysical properties of arylborane–dipyrrinato zinc(ii) complexes†
Abstract
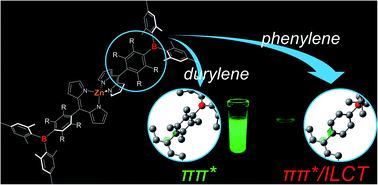
Novel bis(dipyrrinato)zinc(II) derivatives having 4-[bis(2,4,6-trimethylphenyl)boryl]phenyl (ZnBph) or 4-[bis(2,4,6-trimethylphenyl)boryl]-2,3,5,6-tetramethylphenyl groups (ZnBdu) at the 5-position of the dipyrrinato ligands were designed and synthesized. In ZnBph with the smaller dipyrrinate–arylene and arylene–dimesitylboryl dihedral angles, an intramolecular charge transfer arising from the presence of the vacant p orbital on the boron atom participates in the ππ* excited state in character in contrast to the pure ππ* excited state of ZnBdu. The synergistic ππ*/ILCT excited state was weakly fluorescent, and the fluorescence was enhanced upon binding of fluoride to the boron atom.


 Please wait while we load your content...
Please wait while we load your content...