Condensation of pyrylium salts with mixed anhydrides: aryl ethers, aryl amines and sterically congested aromatics†
Abstract
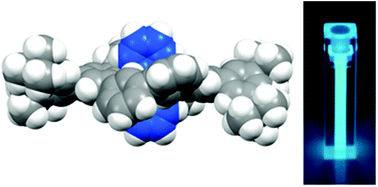
The condensation of easily accessible and widely functionalizable 2,4,6-triaryl pyrylium salts with mixed anhydrides, formed in situ from α-functionalized sodium acetates and an anhydride solvent, leads in good yields to the corresponding 2,4,6-triaryl benzenes functionalized at their 1-position. 4-(Trifluoromethyl)benzoic anhydride has been proven as being the solvent of choice and ortho-disubstituted diphenyl ethers, triphenyl amines and phenyl carbazoles with different functionalizations are synthesized. In addition, sterically hindered anthracene diacetate is condensed with two bis-bromophenyl pyrylium salts which leads after intramolecular Yamamoto coupling to bicyclophanes. In these, the anthracene core is surrounded by phenylenes and protected from photooxidation, leading to a highly stable blue emitting material.


 Please wait while we load your content...
Please wait while we load your content...