Kinetic resolution of azaflavanones via a RuPHOX-Ru catalyzed asymmetric hydrogenation†
Abstract
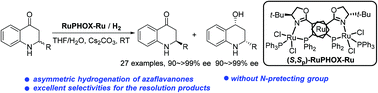
The kinetic resolution of azaflavanones has been established via RuPHOX-Ru catalyzed asymmetric hydrogenation, providing chiral azaflavanones and azaflavanols in high yields with up to >20 : 1 dr and 99.7% ee. The highly efficient kinetic resolution process has been illustrated based on the deuterium labelling and racemization experiments. The reaction could be performed on a gram-scale with a relatively low catalyst loading (2000 S/C), and the resulting products allow for several transformations.


 Please wait while we load your content...
Please wait while we load your content...