Hyperbeanone A, a 5,6-seco-spirocyclic polycyclic polyprenylated acylphloroglucinol derivative with an unprecedented skeleton from Hypericum beanii†
Abstract
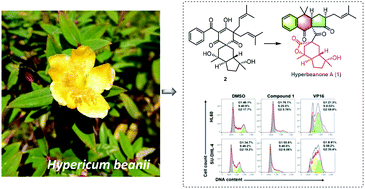
Hyperbeanone A (1), a novel 5,6-seco-polycyclic polyprenylated acylphloroglucinol (PPAP) derivative characterized by an undescribed benz[f]indene-1,9(4H)-dione ring system fused to a tricyclic γ-lactone unit via a ketone carbonyl, together with a known biosynthetic analogue (2), were isolated from the aerial parts of Hypericum beanii. The structure including absolute configuration of 1 was elucidated by extensive analysis of spectroscopic data (mainly including HRESIMS and 1D and 2D NMR) in combination with 13C NMR prediction, calculated 1H/13C NMR-DP4+ probability analyses, and electronic circular dichroism (ECD) methods. The key retro-Claisen, keto–enol, and [4 + 2] cyclization reactions were proposed for the biosynthetic assembly of new scaffold of 1. Compound 1 exhibited specific cytotoxicities against HL-60 (human acute myeloid leukemia) and SU-DHL-4 (diffuse large B-cell lymphoma) cell lines with IC50 values of 7.07 and 12.49 μM, respectively, with no obvious cytotoxicity to MEF (mouse embryonic fibroblast) cells. Compound 1 also induced G1-phase cell cycle arrest and apoptosis in HL-60 and SU-DHL-4 cell lines.


 Please wait while we load your content...
Please wait while we load your content...