A helically twisted ribbon-shaped nanographene constructed around a fenestrindane core†
Abstract
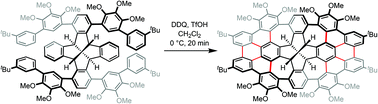
A helically twisted ribbon-shaped nanographene 3 containing four pentagons, eighteen hexagons and four heptagons was synthesized by a cascade of classical Scholl and non-classical Scholl-type cycloheptatriene formation reactions. In the pivotal step of the synthesis, ten carbon–carbon bonds and four cycloheptatriene rings were formed in one single operation in 11% yield. In this way, the fenestrindane core 1 was annelated with two m-quaterphenyl units 4 at opposite rims, generating a unique helically twisted structure 3 with D2-symmetry. X-ray crystallography confirmed this [5.5.5.5]fenestrane-based molecular topology and revealed the aggregation of a pair of antipodal enantiomers, (M)-3 and (P)-3, in the solid state. The torsion angles, planarity of hexagons in the two tribenzo[fg,ij,rst]pentaphene substructures and the local aromaticity of the hexagons were studied based on the crystal data. The optical and electronic properties of compound 3 were investigated by UV/Vis and fluorescence spectroscopy and cyclic voltammetry. Based on the reactions of several partially cyclized intermediates and DFT calculations, a mechanistic sequence of the reaction pathways towards the formation of the nanographene 3 is proposed. In particular, factors that affect the relative ease and the role of hydride abstraction from one of the sp3-hybridized benzhydrylic bridgeheads, giving rise to cycloheptatrien-7-ol side products, are unfolded in this study. It is demonstrated that this side track occurs exclusively at the most highly conjugated π-electron system formed and efficiently competes with the classical and non-classical Scholl cyclodehydrogenation reactions in the last stage of the reaction sequence.


 Please wait while we load your content...
Please wait while we load your content...