Electrochemically site-selective alkoxylation of twisted 2-arylbenzoic acids via spirolactonization†
Abstract
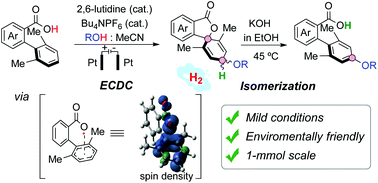
The Electrochemical Cross-Dehydrogenative Coupling (ECDC) of twisted biphenyl-2-carboxylic acids with aliphatic alcohols provides 4′-alkoxyspirolactones which isomerize, under mild basic conditions, to give 4′-alkoxy-2-phenylbenzoic acids. This site-selective alkoxylation was readily adapted to 1 mmol scale and is environmentally friendly, as no terminal oxidants are needed and H2 is the only residue. The suitability of diphenic acid derivatives in this two-step protocol is noteworthy, especially for axially chiral substrates that can be functionalized with retention of the configuration and of the enantiomeric purity. We have proposed a plausible mechanism based on experimental pieces of evidence that support the single-electron oxidation of the carboxylate, formed by deprotonation of the biphenyl-2-carboxylic acids with 2,6-lutidine, and DFT calculations that suggest a very fast spirocyclization of the intermediate σ-aroyloxyl radical. Competing pathways to benzocoumarins were also examined by computational studies.


 Please wait while we load your content...
Please wait while we load your content...