SOMOphilic alkynylation using acetylenic sulfones as functional reagents
Abstract
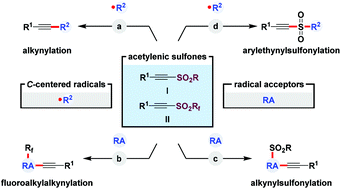
The past decades have witnessed a boom in alkynylation mainly owing to the importance of alkynyl-containing molecules in organic synthesis, drug discovery, polymer chemistry, and materials science. Besides conventional strategies, i.e., nucleophilic and electrophilic alkynylation, SOMOphilic alkynylation has become one of the most straightforward and powerful methods to construct C(sp)–C bonds. This review focuses on the recent developments in the transformations of acetylenic sulfones through a well-known radical addition ensuring C–SO2 bond cleavage relay (α-addition–β-elimination process), where acetylenic sulfones serve as either mono- or multi-functional reagents. In this regard, acetylenic sulfones can directly react with diverse alkyl radical species for the preparation of synthetically useful internal alkynes. On the other hand, acetylenic sulfones undergo a sequence of radical relays including hydrogen atom transfer (HAT), rearrangement, cyclization, SO2 capture, etc. for the simultaneous incorporation of alkynyl and other functionalities. Considering the different reaction modes, these contents employing acetylenic sulfones as functional reagents are divided into four sections: (1) direct alkynylation with various C-centered radicals; (2) alkynylation and fluoroalkylation; (3) alkynylation and sulfonylation; and (4) alkynylation and in situ SO2 capture. Moreover, this review has highlighted the generality of substrate scope and the details of suggested mechanisms.
- This article is part of the themed collection: 2021 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...