Friedel–Crafts acylation of antiaromatic norcorroles: electronic and steric modulation of the paratropic current†
Abstract
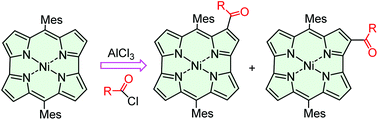
Acyl chlorides react with norcorrolatonickel(II) in dichloromethane in the presence of AlCl3 at room temperature affording two isomeric monoketone derivatives in moderate yields. For acetyl chloride, also diketones were detected and separated. Spectroscopic and electrochemical characterization studies were corroborated using DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...