Supramolecular fluorescence sensing of l-proline and l-pipecolic acid†
Abstract
The current library of synthetic molecular sensors for small polar molecules is limited. In this work, we describe the synthesis of two diastereomeric mono-phosphonate calix[4]pyrrole cavitands, 1in and 1out, acting as fluorescent sensors for amino acids. The two isomeric cavitands differ in the relative orientation, in (1in) and out (1out), of their P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bridging group and the N-phenyl-naphthalamine fluorophore directly attached to it, with respect to their polar aromatic cavities. Using 1H and 31P NMR spectroscopy and non-fluorescent cavitand analogues (5in and 5out), we demonstrate the formation of 1 : 1 complexes with L-proline (L-Pro) and the relevance of the inwardly directed P
O bridging group and the N-phenyl-naphthalamine fluorophore directly attached to it, with respect to their polar aromatic cavities. Using 1H and 31P NMR spectroscopy and non-fluorescent cavitand analogues (5in and 5out), we demonstrate the formation of 1 : 1 complexes with L-proline (L-Pro) and the relevance of the inwardly directed P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the binding of the amino acid. Only the L-Pro⊂5in complex establishes a charged hydrogen bonding interaction between the receptor P
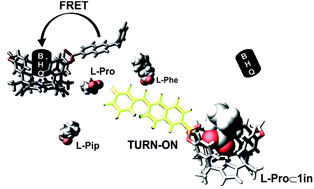
O group in the binding of the amino acid. Only the L-Pro⊂5in complex establishes a charged hydrogen bonding interaction between the receptor P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the protonated amine of the bound zwitterionic amino acid. This interaction is responsible for an increase in the thermodynamic stability of the complex compared to the L-Pro⊂5out counterpart. We investigate the binding properties of the fluorescent cavitands, 1in and 1out, with L-Pro and L-pipecolic acid (L-Pip) at micromolar concentration using emission spectroscopy (direct binding-based sensing, BBS). The observed emission changes in the BBS experiments were small but evidenced the role of the cavitands as fluorescent sensors. In agreement with the millimolar concentration results (1H NMR experiments), the fluorescent 1in sensor displays a larger binding affinity for L-Pro than the 1out isomer. Conversely, the 1out isomer experienced larger emission changes upon amino acid binding. We developed FRET-based indicator displacement assays (IDA) owing to the small emission changes observed in the direct BBS experiments. At micromolar concentration, the competitive displacement of the quencher N-oxide 6 from the cavity of the 1 : 1 supramolecular ensemble (6⊂1in and 6⊂1out), by L-Pro, L-Pip, and L-phenylalanine (L-Phe) produced fluorescence “turn-on”. The results of the BBS and IDA experiments assigned a binding selectivity to the 1in isomer for L-Pro.
O group and the protonated amine of the bound zwitterionic amino acid. This interaction is responsible for an increase in the thermodynamic stability of the complex compared to the L-Pro⊂5out counterpart. We investigate the binding properties of the fluorescent cavitands, 1in and 1out, with L-Pro and L-pipecolic acid (L-Pip) at micromolar concentration using emission spectroscopy (direct binding-based sensing, BBS). The observed emission changes in the BBS experiments were small but evidenced the role of the cavitands as fluorescent sensors. In agreement with the millimolar concentration results (1H NMR experiments), the fluorescent 1in sensor displays a larger binding affinity for L-Pro than the 1out isomer. Conversely, the 1out isomer experienced larger emission changes upon amino acid binding. We developed FRET-based indicator displacement assays (IDA) owing to the small emission changes observed in the direct BBS experiments. At micromolar concentration, the competitive displacement of the quencher N-oxide 6 from the cavity of the 1 : 1 supramolecular ensemble (6⊂1in and 6⊂1out), by L-Pro, L-Pip, and L-phenylalanine (L-Phe) produced fluorescence “turn-on”. The results of the BBS and IDA experiments assigned a binding selectivity to the 1in isomer for L-Pro.
- This article is part of the themed collections: FOCUS: Macrocyclic and supramolecular chemistry and Macrocycle-based Supramolecular Elements


 Please wait while we load your content...
Please wait while we load your content...