The catalytic asymmetric dearomatization of tryptamine for accessing meso-contiguous quaternary carbon centers of oligomeric cyclotryptamine alkaloids: a formal synthesis of hodgkinsine B†
Abstract
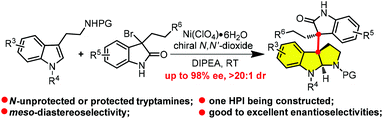
Herein, we disclosed a catalytic asymmetric dearomatization (CADA) of tryptamine via tandem [4 + 2] cycloaddition/cyclization with o-azaxylylene in situ generated from functionalized 3-bromooxindole promoted by chiral N,N′-dioxide/Ni(BF4)2. A variety of dimeric tryptamine derivatives with meso-contiguous quaternary carbon centers were obtained with good to excellent enantioselectivities and diastereoselectivities from Na-unprotected or protected tryptamines. A formal asymmetric synthesis of hodgkinsine B was also executed to showcase its synthetic potential.


 Please wait while we load your content...
Please wait while we load your content...