A solvent-free and efficient synthesis of bicyclic 2-pyridone derivatives for endoplasmic reticulum imaging†
Abstract
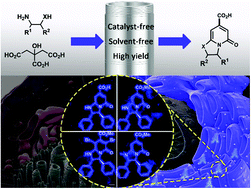
Starting from citric acid (CA) and ethylenediamine derivatives, a solvent-free, catalyst-free and high yielding synthesis approach for bicyclic 2-pyridones is presented. By continuously modifying the core structure, a series of novel and bright fluorophores with outstanding endoplasmic reticulum targeting properties (Pearson's coefficients = 0.89–0.97) and antibacterial activities were obtained. Theoretical calculations and single-crystal structures were further investigated to help reveal the structure–activity relationship. The green preparation method and excellent biological properties make these bicyclic 2-pyridone derivatives very promising for future applications.


 Please wait while we load your content...
Please wait while we load your content...