Recent advances on transition-metal-catalysed asymmetric reductive amination
Abstract
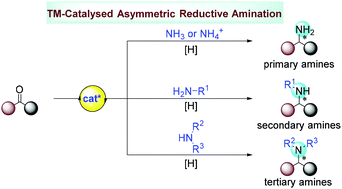
Transition-metal-catalysed asymmetric reductive amination (ARA) represents one of the most straightforward methods to access chiral amines, which converts easily accessible carbonyl compounds (ketones or their derivatives) and versatile ammonia or amines into drug-relevant chiral amines or N-heterocycles in one pot in the presence of a reducing agent and a chiral metal catalyst. The focus of this review is mainly on the progress of homogeneous transition-metal-catalysed ARA reactions with H2 or other hydride sources achieved in the last several years. In addition, the current challenges and potential of this field have been discussed as well.
- This article is part of the themed collection: 2021 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...