A simple removable aliphatic nitrile template 2-cyano-2,2-di-isobutyl acetic acid for remote meta-selective C–H functionalization†
Abstract
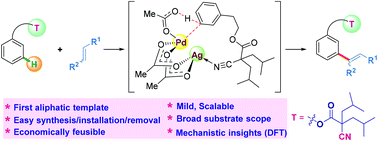
The remote meta-selective C−H bond functionalization of aromatic compounds is one of the most challenging yet valuable aims in synthetic organic chemistry. Despite notable recent achievements, only two types of removable aromatic templates including nitrile based aromatic templates and N-aromatic templates used as directing group templates in the methodologies have been reported so far. No aliphatic “directing group (DG)” template has been developed to date. Herein, we have successfully unveiled a simple and inexpensive removable aliphatic template for the first time as an effective DG template in promoting remote meta-C–H olefination of arenes. Remarkably, the template was obtained in excellent yields in just two steps and without column chromatography purification. The protocol is an efficient, economical, and practical approach in achieving meta-C–H olefination in good to excellent isolated yields with high levels of meta-selectivity under mild conditions. A wide variety of substituted arenes and olefin coupling partners are well tolerated in this reaction. Moreover, the aliphatic template is found to be advantageous due to its easy synthesis, easy installation/removal, and recyclability. We believe that this strategy offers new opportunities for the future development of new DG templates to promote site-selective C–H functionalizations.


 Please wait while we load your content...
Please wait while we load your content...