The catalytic role of elemental sulfur in the DMSO-promoted oxidative coupling of methylhetarenes with amines: synthesis of thioamides and bis-aza-heterocycles†
Abstract
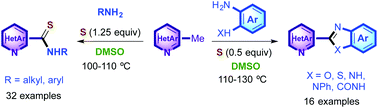
Thioamides could be conveniently synthesized in good to excellent yields via DMSO-promoted oxidative coupling of methylhetarenes with amines in the presence of a near stoichiometric amount of sulfur (1.25 equiv.). Both aliphatic and aromatic amines were found to be competent substrates. When anilines o-substituted by cyclizable groups such as OH, NH2, NHPh, SH and CONH2 were used as amine substrates, the corresponding hybrid bis-aza-heterocycles were formed in high yields even with a sulfur loading as low as 0.5 equiv.


 Please wait while we load your content...
Please wait while we load your content...