Rh(ii)-Catalyzed denitrogenative 1-sulfonyl-1,2,3-triazole-1-alkyl-1,2,3-triazole cross-coupling as a route to 3-sulfonamido-1H-pyrroles and 1,2,3-triazol-3-ium ylides†
Abstract
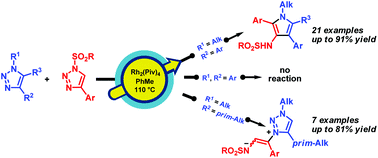
A method for the synthesis of 1-alkyl-3-sulfonamido-1H-pyrroles by the Rh(II)-catalyzed denitrogenative coupling of two different types of 1,2,3-triazoles, 1-alkyl-4-aryl- and 1-sulfonyl-4-aryl-1,2,3-triazoles, has been developed. According to the DFT calculations, the reaction proceeds via the attack of the rhodium-bound azavinyl carbene, derived from the sulfonyl-1,2,3-triazole, at the N2 atom of the 1-alkyl-4-aryl-1,2,3-triazole and the successive formation of the rhodium-bound 1,2,3-triazol-3-ium ylide, metal-free 1,2,3-triazol-3-ium ylide, 1,4,5,8-tetraazaocta-1,3,5,7-tetraene, and 3-(azavinyl)-3,4-dihydro-1,2,4-triazine. The concerted denitrogenative ring contraction of the latter followed by 1,2-prototropic shift affords the 1-alkyl-3-sulfonamidopyrrole. This protocol provides 3-sulfonamidopyrroles from 1-alkyl-4-aryl-1,2,3-triazoles in 24–91% yield. In contrast to 1-alkyl-4-aryl-1,2,3-triazoles, 1,4-dialkyl-1,2,3-triazoles under the same conditions afford stable 1,2,3-triazol-3-ium ylides in 30–99% yield.


 Please wait while we load your content...
Please wait while we load your content...