Generation of powerful organic electron donors by water-assisted decarboxylation of benzimidazolium carboxylates†
Abstract
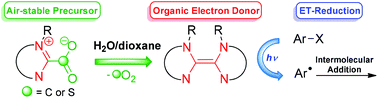
Organic electron donors (OEDs) are increasingly used as efficient reducing agents in various radical reactions. However, their sensitivity to air restraints their practical use and the development of further industrial applications. To meet the community's needs, this paper reports the preparation of air- and moisture-stable carboxylate precursors that can be conveniently activated into the corresponding organic reducer. The new strategy consists in the water-assisted decarboxylation of the adduct at room temperature, providing a straightforward protocol for the single-electron transfer reduction of aryl halides and sulfonamide. The resulting aryl radicals are engaged in the first examples of OED-promoted intermolecular addition reactions. A thorough mechanistic study supports the in situ formation of the organic reducer.


 Please wait while we load your content...
Please wait while we load your content...