Constructing chiral aza-quaternary carbon centers by enantioselective carbonylative Heck reaction of o-iodoanilines with allenes†
Abstract
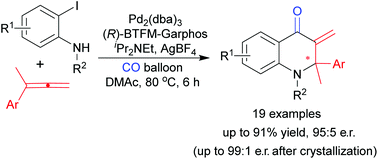
The construction of chiral aza-quaternary carbon centers via C–N bond formation has been achieved by a palladium-catalyzed asymmetric carbonylative Heck reaction of o-iodoanilines with allenes, providing chiral dihydroquinolinone derivatives with moderate to high yield and enantiomeric ratio (er). The er could be improved to 99/1 through the recrystallization of the isolated products. Adding o-iodoaniline into the reaction by syringe pump was crucial to diminish the side reactions of reactive o-iodoanilines and improve the chemoselectivity. Utilizing the chirality induction of an assembled aza-quaternary stereogenic center, molecules bearing vicinal chiral quaternary carbon centers or multiple chiral centers can be rapidly prepared.


 Please wait while we load your content...
Please wait while we load your content...