Abstract
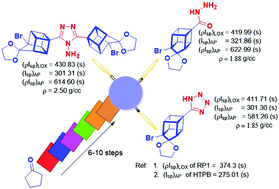
The energetic properties of homocubane derivatives have been evaluated for the first time. The newly synthesized compounds have been fully characterized by spectroscopic methods and single crystal X-ray analysis. Detailed computational studies carried out using the B3LYP/6-311++G(d,p) level of theory reveal that these compounds have higher densities and higher heat of formation as compared to common hydrocarbon fuels. The calculated performances of these compounds in terms of their ballistic properties, particularly density specific impulse (ρIsp), in liquid and in solid propellant systems are substantially superior to those of the conventional fuel RP1 and binder HTPB, respectively. TGA–DTG analysis confirmed that most of these compounds are thermally stable with high onset temperatures. Most of the compounds, except oxadiazole, triazole and triazine containing cages, are also kinetically stable in terms of their HOMO–LUMO energy gap. Quite remarkably, three compounds emerged as excellent candidates for volume-limited applications owing to their high density, density specific impulse as well as kinetic and thermodynamic stabilities.


 Please wait while we load your content...
Please wait while we load your content...