Alkenes as hydrogen trappers to control the regio-selective ruthenium(ii) catalyzed ortho C–H silylation of amides and anilides†
Abstract
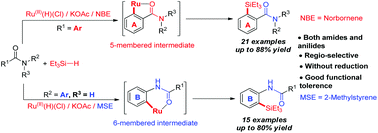
A convenient and practical pathway to versatile silylated amides and anilides is described via efficient and selective ruthenium(II) catalyzed ortho C–H silylation. Both amides and anilides were successfully silylated with good functional group tolerance and high regioselectivity. Different alkenes as the hydrogen acceptors played a crucial role in these two catalytic systems. Unexpectedly, two pathways for RuHCl(CO)(PPh3)2/KOAc catalyzed C–H silylation involving a 5-membered ruthenacycle with arylamides and a 6-membered ruthenacycle with arylanilides, take place depending on the nature of the alkene as the hydrogen trapper.


 Please wait while we load your content...
Please wait while we load your content...