Copper-catalyzed alkynylation/annulation cascades of N-allyl ynamides: regioselective access to medium-sized N-heterocycles†
Abstract
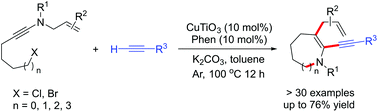
A synthetic strategy based on sequential application of aza-Claisen rearrangement, C–H functionalization, C–N coupling and cyclization as key steps has been developed for the synthesis of various medium-sized N-heterocycles of pharmaceutical relevance. This efficient new method exhibits a broad scope and provides a highly efficient synthesis of N-heterocycles of different ring sizes (6-, 7-, 8-, and 9-membered rings) in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...