Hydrogen bond reinforced, transparent polycaprolactone-based degradable polyurethane†
Abstract
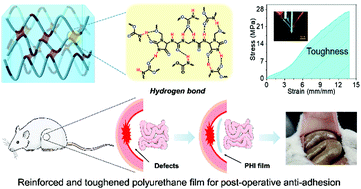
Transparent and degradable polyurethane elastomers with high strength and toughness are in demand for various applications, such as tissue engineering and flexible electronics. However, designing specific chemical structures is challenging, and thus fabricating novel elastomers is sometimes unattainable. An effective approach to develop elastomers is through the introduction of sacrificial bonds, e.g. hydrogen bonds, to enhance their mechanical properties and toughness, which provide hidden lengths and hierarchical structures for energy dissipation. This study introduced a facile and efficient strategy by employing imidazolidinyl urea (IU) as a multiple hydrogen-bonding motif to fabricate transparent and degradable polyurethane elastomers (PHI) with superior breaking strength and excellent toughness. The resultant breaking strength and toughness reached up to 24.9 MPa and 168.2 MJ m−3, respectively. Additionally, the breaking strength increased to 49.9 MPa after the sample was pre-stretched to 600% strain due to strain-induced crystallization (SIC). Moreover, the PHI film with degradability and good biocompatibility showed potential application in post-operative anti-adhesion.
- This article is part of the themed collection: Materials Chemistry in Xi’an Jiaotong University


 Please wait while we load your content...
Please wait while we load your content...