Electrochemical fixation of CO2 over a Mo plate to prepare a Mo2C film for electrocatalytic hydrogen evolution†
Abstract
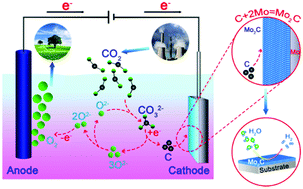
Conversion of carbon dioxide into a hydrogen-evolution electrocatalyst is an ideal protocol to promote carbon neutrality by eliminating emissions of carbon dioxide and producing carbon-free hydrogen energy. Electrochemical fixation of carbon dioxide in molten salts by using a Mo-plate cathode and a Ni–Cr anode is herein demonstrated to produce a cathodic Mo2C film and anodic oxygen. The molten salt electrochemical method offers an efficient modulation of thickness, adhesion and more importantly, interfacial confinement between the Mo2C film and Mo. The resulting Mo2C–Mo binder-free electrode hence shows enhanced electrocatalytic activity towards hydrogen evolution, as rationalized by the lower hydrogen adsorption energy at the Mo2C–Mo interface. The electrochemical reduction of carbon dioxide over a metal substrate is therefore a generic method integrating fixation of carbon dioxide and surface carbonization of a metal to functional films.
- This article is part of the themed collections: FOCUS: Recent progress on electrocatalytic CO2 reduction and 2021 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...