Ferromagnetic Archimedean polyhedra {Fe24M18} (M = Fe, Ni, and Mn) with tunable electron configurations†
Abstract
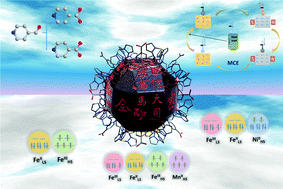
Construction of metal–organic polyhedral clusters is promising and challenging in science due to their highly symmetric structures and potential application to molecular devices. In this work, three neutral nanocages of {[Fe(Tp)(CN)3]24[M(H2O)2]6[M(L)(H2O)]12}·xH2O (1-Fe, 2-Ni, and 3-Mn for M = Fe, Ni, Mn and x = 6, 2, 4, respectively; Tp = hydrotris(pyrazolyl)borate and L = pyridine-4-carboxylate) were prepared through the assembly of [Fe(Tp)(CN)3]− with FeII, NiII, or MnII ions and pyridine-4-carboxaldehyde, which was oxidized to pyridine-4-carboxylic acid during the reactions. Single crystal structure analyses revealed that each cage has the same core structure of an Archimedean polyhedra, the pseudo-rhombicuboctahedron. Elemental analyses and magnetic and spectroscopic measurements were performed to determine the oxidation and spin states of the composite metal ions, demonstrating that their electron configurations can be altered by changing the metal ions and deprotonating the auxiliary ligands. In addition, magnetic studies indicated that the metal ions were ferromagnetically coupled within the cage structure. These clusters exhibited magnetocaloric effects (MCEs) with the entropy changes of 11.55 J kg−1 K−1 for 1-Fe, 5.84 J kg−1 K−1 for 2-Ni, and 19.84 J kg−1 K−1 for 3-Mn at 3 K and 8.5 T, and 3-Mn is among the family of high-performance 3d-metal-based magnetic coolants.


 Please wait while we load your content...
Please wait while we load your content...