CNT-modified two-phase manganese hexacyanoferrate as a superior cathode for sodium-ion batteries†
Abstract
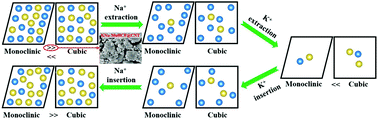
The manganese-based Prussian blue and its analogues (PBAs) are regarded as promising cathode materials for sodium-ion batteries (SIBs). However, the effective suppression of Fe(CN)6 vacancies and removal of the absorbed and interstitial water in their crystal structures are two critical points to achieve excellent electrochemical performances. In this study, carbon nanotubes (CNT) were employed to synthesize the hybrid KNa-MnFe(CN)6@CNT material via a facial concentration-gradient coprecipitation method. The systematic characterization of this material revealed that the water content was effectively reduced and a two-phase (monoclinic and cubic) structure was obtained. The well-designed hybrid KNa-MnFe(CN)6@CNT electrode exhibited an outstanding electrochemical performance with a very high initial discharge capacity of 164.5 mA h g−1 (ICE = 96.3%) and high capacity retention of 82.0% after 100 cycles at the current density of 20 mA g−1 in the voltage range of 2.0–4.2 V. In addition, a high rate performance of 77.3 mA h g−1 was achieved at a current density of 1 A g−1 due to the good adhesion between the PBA particles and CNT. The good structural reversibility of the KNa-MnFe(CN)6@CNT electrode was confirmed during the extraction/insertion process of alkali metal ions. Thus, this work provides a prospective strategy to develop high-performance hybrid manganese-based PBA cathode materials for SIBs.


 Please wait while we load your content...
Please wait while we load your content...