A low-temperature oxyl transfer to carbon monoxide from the ZnII–oxyl site in a zeolite catalyst
Abstract
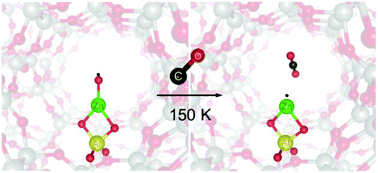
An atomic O radical anion bound to a metal ion (metal–oxyl) is a key intermediate in a variety of oxidative reactions. Understanding its structure–reactivity relationship is highly desirable for the rational design of challenging oxidative transformation processes. However, due to the difficulty of analysis, even the identification of an oxyl is a challenging subject especially in the research field of heterogeneous catalysts. Here, we report for the first time a low-temperature oxyl transfer to CO from the ZnII–oxyl bond isolated in a zeolite catalyst. Zeolite matrix isolation of this novel ZnII–oxyl bond allows us to observe the unique spectroscopic probes of the oxyl: a vibronically-resolved spectrum and ESR signatures. Using the oxyl-selective spectroscopic probes, we successfully demonstrated that the ZnII–oxyl bond has the capability of transferring the oxyl to CO even at 150 K with the generation of a single ZnI˙ species. The superhyperfine interaction of the ZnI˙ species with the framework Al atom, observed during the oxyl-transfer reaction, provided direct experimental evidence that the oxyl-functionality emerged at the framework Al site. DFT calculations showed that the ZnII–oxyl bond, which is constrained by the zeolite lattice ligation, acts as a superior electron donor toward CO at the rate-determining step of the oxyl-transfer reaction and effectively reduces the barrier to be <5 kJ mol−1. Based on the results obtained in the present study as well as the previous work, we further deepen the understanding of why the abnormal ZnII–oxyl bond having exceptional reactivities is formed by the zeolite lattice ligation.


 Please wait while we load your content...
Please wait while we load your content...
