A hydrolytically stable Ce(iv) complex of glutarimide-dioxime†
Abstract
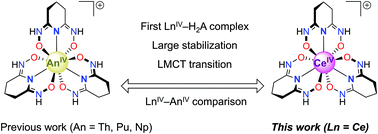
The coordination chemistry of glutarimide-dioxime (H3A) has been studied related to applications in uranyl sequestration from seawater and for the stabilization of early transition metals in high oxidation states. We report here that the H2A− anion is also suitable for stabilizing Ce(IV) and acts as a tridentate ligand to form the [Ce(H2A)3]+ cation. The metal complexes [Ce(H2A)3]Cl and [Ce(H2A)3][BPh4] have been obtained by oxidation of CeIII in the presence of H3A under aerobic conditions. UV-Vis spectroscopy and DFT calculations were performed to characterize the electronic structure and ligand-to-metal charge transfer (LMCT) bands of [CeIV(H2A)3]+. X-ray absorption spectroscopy (XAS) was also performed to verify the Ce(IV) oxidation state. Absent a clear electrochemical signal for cerium reduction in [Ce(H2A)3]Cl or [Ce(H2A)3][BPh4] under a range of conditions, DFT calculations predicted a Ce(III/IV) redox couple of −1.22 V vs. Fc/Fc+. These results further expand the coordination chemistry of glutarimide-dioxime to tetravalent cerium.
- This article is part of the themed collection: Rare Earth Chemistry – In memory of Professor Xu Guangxian at his centenary


 Please wait while we load your content...
Please wait while we load your content...