Luminescence property switching in 1D supramolecular polymerization of organic donor–π-acceptor chromophores†
Abstract
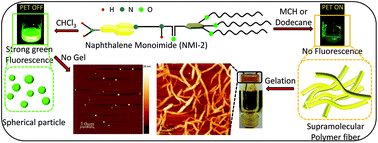
The role of solvents in the control over supramolecular interactions has been studied for decades in protein folding, soft material fabrication and the long-range organization of functional organic π chromophores. Nonpolar co-solvents (poor solvents) are commonly used to initiate supramolecular polymerization in organic solvents. It is very essential to gain more insight into the understanding of the processing methodology. Hence, to investigate the influence of the nonpolar co-solvent composition on supramolecular polymerization initiation and chain propagation, a fluorophore-spacer-receptor type naphthalimide derivative (NMI-2) has been synthesized. With the change of good solvent to poor solvent ratio, NMI-2 exhibits supramolecular polymerization in a J-type fashion leading to 1D-nanowires as demonstrated by spectroscopic and microscopic studies. The efficient gel formation in non-polar solvents is evidenced by microscopic and rheological data. Analysis of co-solvent composition, temperature variable spectroscopic data and the methanol experiment reveals the crucial role of a critical co-solvent composition (CCSC) to initiate the polymerization process and H-bonding followed by π-stacking interactions to propagate the polymer chain in a long-range order. A visual color change due to a red-shift of the intramolecular charge transfer (ICT) band and fluorescence turn-off phenomenon as a result of photoinduced electron transfer (PET) from the aryl receptor to the naphthalimide fluorophore during the initiation of supramolecular polymerization is denoted as the indicator of supramolecular polymerization initiation. The observed phenomenon is supported by extensive atomic force microscopy (AFM) studies and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...