Influence of the tetraalkoxysilane crosslinker on the properties of polysiloxane-based elastomers prepared by the Lewis acid-catalysed Piers-Rubinsztajn reaction†
Abstract
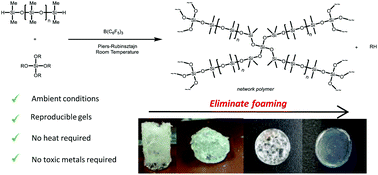
We investigate the preparation of polysiloxane-based networks under solvent-free, ambient conditions using the Lewis acid catalysed Piers-Rubinsztajn (PR) reaction of hydride-terminated siloxanes with various tetrafunctional alkoxysilanes (tetraethoxysilane, tetrapropoxysilane, tetra-n-buxoxysilane, tetra-s-butoxysilane, tetra-s-butoxysilane, and tetrakis(2-ethylbutoxy)silane) as crosslinkers. We explore the effects of polysiloxane chain length and crosslinker alkyl group on the rheological performance of the elastomers. By analysing the reaction progress by grazing angle Fourier-transform infrared spectroscopy (FTIR) and determining the rheological properties of the resulting materials, we show that the use of linear or branched alkoxysilanes strongly influences the morphology and properties of these network polymers. We have shown the PR process can be tailored to reliably produce homogeneous, polysiloxane network materials. This work provides information on the relative rates of network formation under ambient conditions with an emphasis on the impact of crosslinker alkyl chain length. Our results show that electronics and sterics both play critical roles in influencing the rate of the curing reaction. Crucially, we newly demonstrate the benefit of a having tertiary carbon α to the SiO reaction centre, as is the case for the tetra-s-butoxysilane crosslinker, for delivering exceptionally rapid network cure and a concomitant enhancement in storage modulus of the resultant materials.


 Please wait while we load your content...
Please wait while we load your content...