Reversible polycondensations outside the Jacobson–Stockmayer theory and a new concept of reversible polycondensations†
Abstract
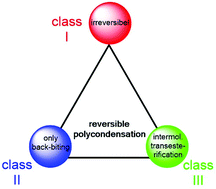
L-Lactide was polymerized with tin(II)acetate, tin(II)2-ethyl hexanoate, diphenyltin dichloride and dibutyltin bis(pentafluorophenoxide) at 130 °C in bulk. When an alcohol was added as initiator, linear chains free of cycles were formed having a degree of polymerization (DP) according to the lactide/initiator (LA/In) ratio. Analogous polymerizations in the absence of an initiator yielded high molar mass cyclic polylactides. Quite similar results were obtained when ε-caprolactone was polymerized with or without initiator. Several transesterification experiments were conducted at 130 °C, either with polylactide or poly(ε-caprolactone) indicating that several transesterification mechanisms are operating under conditions that do not include formation of cycles by back-biting. Furthermore, reversible polycondensations (revPOCs) with low or moderate conversions were found that did not involve any kind of cyclization. Therefore, these results demonstrate the existence of revPOCs, which do neither obey the theory of irreversible polycondensation as defined by Flory nor the hypothesis of revPOCs as defined by Jacobson and Stockmayer. A new concept encompassing any kind of revPOCs is formulated in the form of a “polycondensation triangle”.


 Please wait while we load your content...
Please wait while we load your content...