Supramolecular organogel formation behaviors of beads-on-string shaped poly(azomethine)s dependent on POSS structures in the main chains†
Abstract
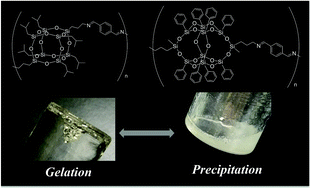
Beads-on-string-shaped polymers incorporating cage silsesquioxanes in the main chain, especially double-decker-shaped phenyl-substituted silsesquioxanes (DDSQ), have received significant attention in the past few decades. Difunctionalized isobutyl-substituted cage octasilsesquioxanes (T8) have also been generating this type of polymer architecture. However, direct comparisons between main-chain-type polymers comprising phenyl-substituted DDSQ and isobutyl-substituted T8 units have seldom been reported. In this study, we prepared bis(3-aminopropyl)-DDSQ (6) via the hydrosilylation of 3,13-dihydrooctaphenylhexacyclodecasiloxane (dihydro-DDSQ) with t-butyl N-allylcarbamate and deprotection with trifluoroacetic acid. Both para-bis(3-aminopropyl)hexaisobutyl-substituted T8 cage (1), which was developed by us, and 6 were polymerized with terephthalaldehyde (2a), isophthalaldehyde (2b), and 4,4′-oxydibenzaldehyde (2c) to produce the corresponding T8-poly(azomethine)s (3a–c) and DDSQ-poly(azomethine)s (7a–c), respectively. Although precipitates were observed when the polymerization solution of 7 was concentrated under reduced pressure, 3 underwent organogel formation. Solubility test results for heptaisobutyl–hydride–POSS (8) and dihydro-DDSQ (9) as model compounds for the POSS units in 3 and 7, respectively, suggested that the wider solubility spectrum of the isobutyl-substituted T8 moieties in 3 may provide a suitable balance for organogel formation, and the narrower spectrum of solubility for the phenyl-substituted DDSQ moieties in 7 may prevent the existence of a specific solvent region for suitable balanced gel formation. The concentration (c) dependences of zero shear relative viscosity (η0,r) for the polymerization solutions of 3a–c, containing various linker structures were well described by an equation with a single value of the characteristic exponent using different values of gelation threshold concentration.


 Please wait while we load your content...
Please wait while we load your content...