Higher-order interfiber interactions in the self-assembly of benzene-1,3,5-tricarboxamide-based peptides in water†
Abstract
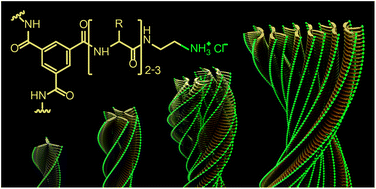
Mimicking the complexity of biological systems with synthetic supramolecular materials requires a deep understanding of the relationship between the structure of the molecule and its self-assembly pattern. Herein, we report a series of water-soluble benzene-1,3,5-tricarboxamide-based di- and tripeptide derivatives modified with small non-bulky terminal amine salt to induce self-assembly into twisted one-dimensional higher-order nanofibers. The morphology of nanofibers strongly depends on the nature, order, and quantity of amino acids in the short peptide fragments and vary from simple cylindrical to complex helical. From observations of several fiber-splitting events, we detected interfiber interactions that always occur in a pairwise manner, which implies that the C3 symmetry of benzene-1,3,5-tricarboxamide-based molecules in higher-order fibers becomes gradually distorted, thus facilitating hydrophobic contact interactions between fibrils. The proposed mechanism of self-assembly through hydrophobic contact allowed the successful design of a compound with pH-responsive morphology, and may find use in the future development of complex hierarchical architectures with controlled functionality.


 Please wait while we load your content...
Please wait while we load your content...