Construction of biodegradable core cross-linked nanoparticles from near infrared dyes encoded in polyprodrug amphiphiles and investigation of their synergistic anticancer activity†
Abstract
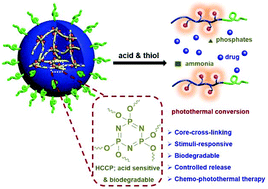
It has been widely accepted that covalently cross-linked nanoparticles show extended circulation time in the blood due to their large stable structures compared to free linear polyprodrugs. Herein, an efficient method, combining living radical polymerization, a ring-opening reaction, and a copper(I) catalyzed azide–alkyne cycloaddition click reaction, was employed to prepare amphiphilic polyprodrugs with a reduction-responsive camptothecin (CPT) prodrug and photothermal converted near-infrared dyes (IR780). The drug content could be regulated as needed through the monomer feed ratio. The obtained amphiphilic P[CPTM-co-GMA(-IR780/-OH)]-b-POEGMA polyprodrugs could molecularly dissolve in a good solvent and self-assembled into nanoparticles consisting of thiol-responsive P[CPTM-co-GMA(-IR780/-OH)] cores and well solvated POEGMA coronas in the selected solvent. Cross-linking of the P[CPTM-co-GMA(-IR780/-OH)] cores could be facilely achieved via the reaction between the residual hydroxyls and hexachlorocyclic triphosphonitrile (HCCP; acid sensitive and biodegradable) to form structurally stable core cross-linked (CCL) nanoparticles. DLS, TEM, UV-vis and fluorescent techniques, as well as subsequent in vitro cell experiments, confirmed that these CCL nanoparticles possessed excellent aqueous stability, high tumor micromilieu sensitivity, controlled drug release and severe cytotoxicity to HeLa cells with the aid of near-infrared light irradiation. Not only that, these CCL nanoparticles could serve as nanocarriers for loading other anti-cancer drugs (curcumin) to realize synergistic and highly effective treatment. Overall, this work provides a convenient way to produce promising intelligent, responsive and enhanced anticancer therapies.


 Please wait while we load your content...
Please wait while we load your content...