Base-promoted thioannulation of o-alkynyl oxime ethers with sodium sulfide for the general synthesis of isothiocoumarins†
Abstract
A general and efficient strategy for the one-pot synthesis of isothiocoumarin-1-ones has been developed via the base-promoted 6-endo-dig thioannulation of o-alkynyl oxime ethers using the cheap and readily available Na2S as the sulfur source. Mechanistic studies disclosed that the reaction proceeded through two C–S bond formations, N–O bond cleavage and the final hydrolysis of imines.
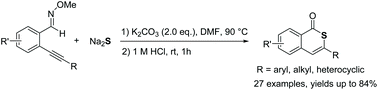


 Please wait while we load your content...
Please wait while we load your content...