Predictable site-selective functionalization: Promoter group assisted para-halogenation of N-substituted (hetero)aromatics under metal-free condition†
Abstract
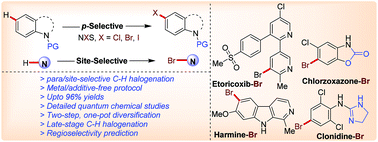
Herein, regioselective para-C–H halogenation of N-pyrimidyl (hetero)aromatics through SEAr (electrophilic aromatic substitution) type reaction is disclosed. SEAr type reaction has been utilized for the C5-bromination of indolines (para-selective) with N-bromosuccinimide under metal and additive-free conditions in good to excellent yields. The developed methodology is also applicable for iodination and challenging chlorination. The pyrimidyl group is identified as a reactivity tuner that also controls the regioselectivity. The present method is also applicable for selective halogenation of aniline, pyridine, indole, oxindole, pyrazole, tetrahydroquinoline, isoquinoline, and carbazole. DFT studies such as Fukui nucleophilicity and natural charge maps also support the observed p-selectivity. Post-functionalization of the title compound into the corresponding arylated, olefinated, and dihalogenated products is achieved in a one-pot, two-step fashion. Late-stage C–H bromination was also executed on drug/natural molecules (harmine, etoricoxib, clonidine, and chlorzoxazone) to demonstrate the applicability of the developed protocol.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...