Enantioselective hydrophosphonylation of N-Boc imines using chiral guanidine–thiourea catalysts†
Abstract
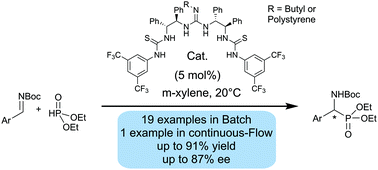
The enantioselective hydrophosphonylation of N-Boc imines was investigated using a new family of pseudo-symmetric guanidine–thiourea catalysts, providing α-amino phosphonates in moderate to high yields with good enantioselectivity. The catalyst was heterogenized by polymerization with styrene and the resulting catalyst was applied to reactions under continuous-flow conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...