Control of RNA with quinone methide reversible acylating reagents†
Abstract
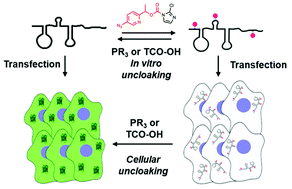
Caging RNA by polyacylation (cloaking) has been developed recently as a simple and rapid method to control the function of RNAs. Previous approaches for chemical reversal of acylation (uncloaking) made use of azide reduction followed by amine cyclization, requiring ∼2–4 h for the completion of cyclization. In new studies aimed at improving reversal rates and yields, we have designed novel acylating reagents that utilize quinone methide (QM) elimination for reversal. The QM de-acylation reactions were tested with two bioorthogonally cleavable motifs, azide and vinyl ether, and their acylation and reversal efficiencies were assessed with NMR and mass spectrometry on model small-molecule substrates as well as on RNAs. Successful reversal both with phosphines and strained alkenes was documented. Among the compounds tested, the azido-QM compound A-3 displayed excellent de-acylation efficiency, with t1/2 for de-acylation of less than an hour using a phosphine trigger. To test its function in RNA caging, A-3 was successfully applied to control EGFP mRNA translation in vitro and in HeLa cells. We expect that this molecular caging strategy can serve as a valuable tool for biological investigation and control of RNAs both in vitro and in cells.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...