Oxazaborolidine-catalyzed reductive parallel kinetic resolution of ketones from β-nitro-azabicycles for the synthesis of chiral hypoestestatins 1, 2†
Abstract
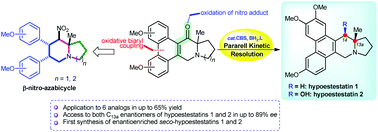
A novel approach for the synthesis of 13a-methyl tylophora alkaloids has been reported. The key features included two different synthetic pathways targeted at transforming the β-nitro-azabicycle to the phenanthrene core. The successful steps involved the oxidation of the nitro-piperidine moiety to the corresponding α,β-unsaturated ketone, and an oxidative biaryl coupling reaction for phenanthrene ring formation. Finally, the desired product was obtained via a formal reductive removal of the hydroxyl group. This methodology has been applied for the synthesis of 13a-methyl tylophora alkaloids in up to 65% yield over six steps from β-nitro-azabicycles. Both natural and unnatural enantioenriched hypoestestatins 1 and 2, and related compounds were synthesized using parallel kinetic resolution of the CBS-oxazaborolidine-catalyzed reduction of racemic ketones to provide two separable diastereomeric alcohols in combined yields up to 91% and with high enantioselectvity (up to 89% ee). In addition, the catalytic asymmetric reduction to seco-hypoestestatins 1 and 2 has been reported for the first time. Thus, the ability to develop the racemic mixtures to both enatioenriched forms offers benefit for various biological assays in the future.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...