1,2-Bis-perfluoroalkylations of alkenes and alkynes with perfluorocarboxylic anhydrides via the formation of perfluoroalkylcopper intermediates†
Abstract
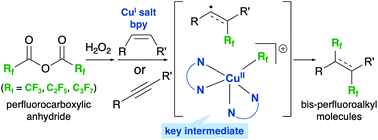
A novel, Cu-mediated protocol toward the 1,2-bis-perfluoroalkyaltion of alkenes/alkynes was developed. The method proceeded with perfluorocarboxylic anhydrides as inexpensive and readily available perfluoroalkyl sources. Diacyl peroxide was generated in situ from the perfluorocarboxylic anhydrides and H2O2. The key step in this reaction is the formation of a stable perfluoroalkylcopper intermediate that is achieved with the aid of a bipyridyl ligand. Subsequent reaction of the intermediate with perfluoroalkyl-containing alkyl or vinyl radicals affords the desired products.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...