Double asymmetric intramolecular aza-Michael reaction: a convenient strategy for the synthesis of quinolizidine alkaloids†
Abstract
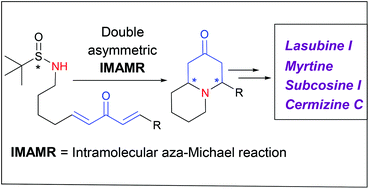
A new methodology to access the quinolizidine skeleton in an asymmetric fashion was devised. It involves two consecutive intramolecular aza-Michael reactions of sulfinyl amines bearing a bis-enone moiety, in turn generated by a monodirectional cross metathesis reaction. The sequence, which takes place with excellent yields and diastereocontrol, was applied to the total synthesis of alkaloids lasubine I and myrtine.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...