Synthesis of 3-nitroindoles by sequential paired electrolysis†
Abstract
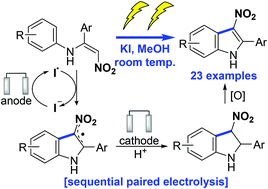
3-Nitroindoles are synthetically versatile intermediates but current methods for the preparation hinder their widespread application. Herein, we report that nitroenamines undergo electrochemical cyclisation to 3-nitroindoles in the presence of potassium iodide. Detailed control experiments and cyclic voltammogram studies infer the reaction proceeds via a sequential paired electrolysis process, beginning with anodic oxidation of iodide (I−) to the iodine radical (I˙), which facilitates cyclisation of the nitroenamine to give a 3-nitroindolinyl radical. Cathodic reduction and protonation generates a 3-nitroindoline that upon oxidation forms the 3-nitroindole.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...