Calculated oxidation potentials predict reactivity in Baeyer–Mills reactions†
Abstract
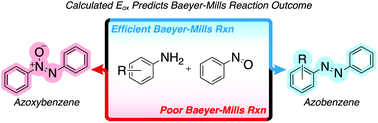
Azobenzenes are widely used as dyes and photochromic compounds, with the Baeyer–Mills reaction serving as the most common method for their preparation. This transformation is often plagued by low yields due to the formation of undesired azoxybenzene. Here, we explore electronic effects dictating the formation of the azoxybenzene side-product. Using calculated oxidation potentials, we were able to predict reaction outcomes and improve reaction efficiency simply by modulating the oxidation potential of the arylamine component.


 Please wait while we load your content...
Please wait while we load your content...