Solvent-directed formation of helically twisted stacking constructs via self-assembly of tris(phenylisoxazolyl)benzene dimers†
Abstract
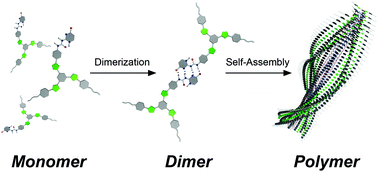
Ureido-pyrimidinone (UPy)-appended tris(phenylisoxazolyl)benzenes were synthesized. The UPy moieties of the tris(phenylisoxazolyl)benzenes stably formed self-complementary dimers in solution. The dimers self-assembled to form helically twisted stacking constructs in a process driven by π–π stacking interactions of UPy dimer moieties and dipole–dipole interactions of isoxazole units. Strong association affinity was seen within the stacking constructs compared with the previously reported isoxazole derivatives owing to the auxiliary π–π stacking interaction. Notably, tris(phenylisoxazolyl)benzenes showed an environmentally responsive nature. The absorption bands, emission intensities, and sizes of ensembles depended significantly on the mixing ratio of CHCl3 and methylcyclohexane (MCH). Additionally, sharp on–off switching phenomena were seen in their circular dichroism (CD) and circularly polarized luminescence (CPL) spectra in response to the mixing ratio of CHCl3 and MCH. CD and CPL were activated only at a certain mixing ratio of CHCl3/MCH, thus showing potential for the creation of molecular sensors.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...