Asymmetric Michael reaction of 3-homoacyl coumarins with chromone-fused dienes toward enantioenriched coumarin chromone skeletons†
Abstract
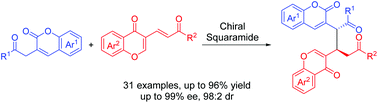
Asymmetric Michael reaction of 3-homoacyl coumarins and chromone-fused dienes was developed by employing a chiral squaramide, and a series of coumarin chromone skeletons were furnished in moderate to high yields (up to 99%) and stereoselectivities (up to 98 : 2 dr, 99% ee).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...