A metal-free Petasis reaction towards the synthesis of N-(α-substituted)alkyl sulfoximines/sulfonimidamides†
Abstract
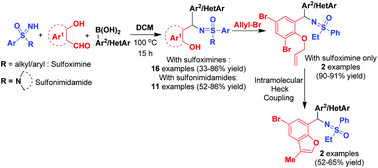
Herein, we disclose a metal-free novel approach for the synthesis of N-(α-substituted)alkyl sulfoximines/sulfonimidamides via one-pot multicomponent Petasis reactions of aryl boronic acids, ortho-hydroxyarylaldehydes and sulfoximines/sulfonimidamides in moderate to very good yields. The presence of two chiral centres provides a mixture of diastereomers almost in a 1 : 1 ratio, which are separated successfully in most of the cases. The –OH functionality of Petasis products is further utilized to derive heterocycles via O-allylation, followed by intramolecular Heck cyclization, proving the synthetic utility of the products.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...